Hypochlorous acid is a remarkable compound that plays a crucial role in various applications, from healthcare to sanitation. Its simple yet effective chemical formula, HOCl, has garnered significant attention due to its potent antibacterial and antiviral properties. This versatile acid is naturally produced by the human immune system, where it serves as a powerful defense mechanism against harmful pathogens.
With its increasing popularity, the hypochlorous acid formula is being widely researched and developed for numerous uses. Its eco-friendly nature and non-toxic properties make it a preferred choice for many industries, including healthcare, food safety, and water treatment. This article delves into the intricacies of hypochlorous acid, exploring its chemical structure, benefits, and various applications in our daily lives.
As we navigate through this comprehensive guide, we aim to provide a clear understanding of the hypochlorous acid formula, its characteristics, and the myriad ways it is utilized. Whether you're a student, a professional, or simply curious about chemistry, this article will offer valuable insights into this fascinating compound and its significant impact on modern science and technology.
Read also:Ultimate Guide To Kannada Movierulz 2023 Everything You Need To Know
Table of Contents
- What is Hypochlorous Acid?
- Chemical Structure of Hypochlorous Acid
- How is Hypochlorous Acid Produced?
- Natural Production in the Human Body
- Industrial Production Methods
- Why is Hypochlorous Acid Important?
- Antimicrobial Properties
- Applications of Hypochlorous Acid
- Uses in Healthcare
- Role in Food Safety
- Water Treatment Solutions
- Environmental Impact
- Safety and Handling
- Common Questions about Hypochlorous Acid
- Conclusion
What is Hypochlorous Acid?
Hypochlorous acid (HOCl) is a weak acid that is part of the chlorine family of compounds. It is a clear, colorless solution that is renowned for its disinfecting abilities. As a naturally occurring substance, hypochlorous acid is produced by white blood cells in mammals as part of the body's immune response. Its primary function is to combat pathogens such as bacteria, viruses, and fungi, making it an indispensable component of the immune system.
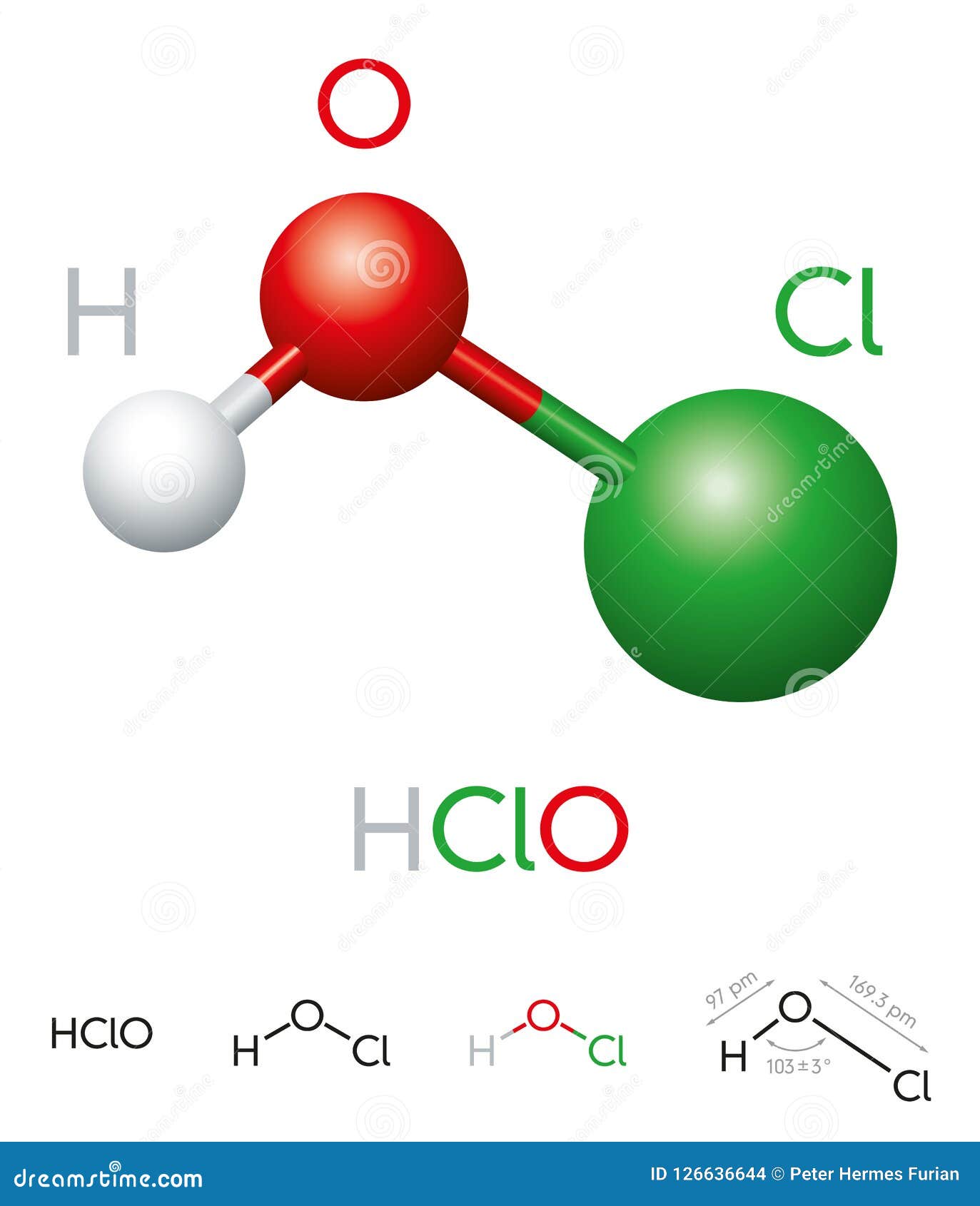
In addition to its biological role, hypochlorous acid is synthesized for various industrial applications. Its effectiveness as a sanitizer and disinfectant is well-documented, and it is used extensively in medical, food, and water treatment industries. The chemical formula of hypochlorous acid, HOCl, is indicative of its composition, consisting of one hydrogen atom, one chlorine atom, and one oxygen atom.
Chemical Structure of Hypochlorous Acid
The chemical structure of hypochlorous acid is relatively simple, yet it is this simplicity that contributes to its effectiveness. The formula HOCl represents its molecular composition, where the hydrogen atom is bonded to oxygen, which is in turn bonded to chlorine. This structure enables hypochlorous acid to act as a powerful oxidizing agent, disrupting the cell walls of microorganisms and rendering them inactive.
Furthermore, the molecule's polar nature allows it to penetrate lipid membranes easily, enhancing its efficacy as a disinfectant. This property is particularly beneficial in healthcare settings, where hypochlorous acid is used to sanitize surfaces and equipment, ensuring a sterile environment free from harmful pathogens.
How is Hypochlorous Acid Produced?
Hypochlorous acid can be produced both naturally and industrially. In the human body, it is generated by white blood cells through a process called the respiratory burst. This involves the reaction of hydrogen peroxide with chloride ions, catalyzed by the enzyme myeloperoxidase, resulting in the formation of hypochlorous acid. This biological production is crucial for the immune system's ability to fight off infections and maintain health.
Natural Production in the Human Body
The natural production of hypochlorous acid in the human body is an essential part of the immune response. When pathogens invade, white blood cells, particularly neutrophils, are activated and release an enzyme called myeloperoxidase. This enzyme facilitates the conversion of hydrogen peroxide and chloride ions into hypochlorous acid, which then attacks and neutralizes the invading microorganisms.
Read also:All You Need To Know About Crosshatched Parking Rules Benefits And Tips
This natural defense mechanism is highly efficient and is one of the reasons why hypochlorous acid is considered a safe and effective disinfectant for use in various settings. Its non-toxic properties make it an ideal choice for applications where human exposure is a concern, such as in wound care and surface disinfection.
Industrial Production Methods
Industrially, hypochlorous acid is produced through the electrolysis of a saltwater solution. This process involves passing an electric current through the solution, causing the chloride ions to react with water and form hypochlorous acid. This method is widely used due to its efficiency and the ability to produce large quantities of hypochlorous acid for commercial purposes.
Electrolysis is a clean and sustainable method of production, as it requires only salt, water, and electricity. The resulting hypochlorous acid is effective as a disinfectant and is used in various industries, including healthcare, agriculture, and food processing. Additionally, the by-products of this process are environmentally friendly, making it an attractive option for eco-conscious industries.
Why is Hypochlorous Acid Important?
Hypochlorous acid plays a vital role in both natural and industrial contexts due to its potent antimicrobial properties. Its importance stems from its ability to effectively eliminate a wide range of pathogens, making it a valuable tool in infection control and prevention. In healthcare settings, hypochlorous acid is used to sanitize surfaces, equipment, and even wounds, reducing the risk of infections and promoting healing.
Antimicrobial Properties
The antimicrobial properties of hypochlorous acid are a result of its oxidizing nature, which allows it to disrupt the cell walls of bacteria and viruses. This action not only kills the microorganisms but also prevents their replication, effectively halting the spread of infections. The versatility of hypochlorous acid makes it suitable for use against a broad spectrum of pathogens, including antibiotic-resistant strains.
Furthermore, its rapid action and low toxicity make it an ideal choice for disinfection in environments where human contact is inevitable. Unlike traditional disinfectants, hypochlorous acid does not leave harmful residues and does not contribute to the development of resistant strains, making it a sustainable solution for long-term hygiene management.
Applications of Hypochlorous Acid
The applications of hypochlorous acid are vast and varied, thanks to its unique properties. From healthcare to agriculture, this versatile compound is utilized in numerous industries to enhance safety and hygiene standards. Its effectiveness as a disinfectant and sanitizer is well-recognized, leading to its widespread adoption in various sectors.
Uses in Healthcare
In healthcare, hypochlorous acid is employed for its powerful disinfection capabilities. It is used to sanitize surfaces, medical instruments, and even the skin, reducing the risk of infections and ensuring a sterile environment. Its non-toxic nature makes it safe for use in wound care, where it promotes healing by eliminating bacteria and other pathogens without damaging healthy tissue.
Hypochlorous acid is also used in healthcare settings for environmental disinfection, helping to prevent the spread of hospital-acquired infections. Its ability to effectively eliminate a wide range of microorganisms, including antibiotic-resistant strains, makes it an invaluable tool in maintaining a clean and safe healthcare environment.
Role in Food Safety
In the food industry, hypochlorous acid is used to enhance food safety by disinfecting surfaces, equipment, and even the food itself. Its ability to eliminate bacteria, viruses, and fungi ensures that food products are free from harmful pathogens, reducing the risk of foodborne illnesses.
Hypochlorous acid is also used in the processing and packaging of food products, where it helps to maintain hygiene standards and extend the shelf life of perishable goods. Its non-toxic properties make it safe for use in food environments, ensuring that food products remain safe for consumption while minimizing the risk of contamination.
Water Treatment Solutions
In water treatment, hypochlorous acid is used to disinfect drinking water and wastewater, ensuring that it is free from harmful microorganisms. Its effectiveness as a biocide makes it a preferred choice for water treatment facilities, where it helps to maintain safe and clean water supplies for communities.
Hypochlorous acid is also employed in swimming pool maintenance, where it helps to keep the water clean and free from pathogens. Its ability to effectively eliminate bacteria and viruses ensures that swimming pools remain safe for public use, reducing the risk of waterborne infections and illnesses.
Environmental Impact
The environmental impact of hypochlorous acid is minimal, thanks to its non-toxic and biodegradable nature. Unlike traditional disinfectants, hypochlorous acid does not contribute to pollution or harm the environment, making it a sustainable choice for industries looking to reduce their ecological footprint.
Hypochlorous acid breaks down into harmless by-products, such as water and salt, after use, ensuring that it does not leave behind harmful residues. This property makes it an attractive option for eco-conscious industries and individuals looking to maintain hygiene without compromising the environment.
Safety and Handling
When handling hypochlorous acid, it is essential to follow safety guidelines to ensure safe and effective use. Although it is non-toxic and generally safe for human exposure, it is still important to handle it with care to prevent any potential irritation or adverse reactions.
When using hypochlorous acid as a disinfectant or sanitizer, it is recommended to wear protective gloves and eyewear to prevent contact with the skin and eyes. Additionally, it is important to store hypochlorous acid in a cool, dry place away from direct sunlight to preserve its effectiveness and prevent degradation.
Common Questions about Hypochlorous Acid
What is the chemical formula of hypochlorous acid?
The chemical formula of hypochlorous acid is HOCl. It consists of one hydrogen atom, one oxygen atom, and one chlorine atom.
Is hypochlorous acid safe for use on skin?
Yes, hypochlorous acid is non-toxic and generally safe for use on skin. It is often used in wound care and as a skin disinfectant due to its antimicrobial properties.
How does hypochlorous acid work as a disinfectant?
Hypochlorous acid works as a disinfectant by oxidizing and disrupting the cell walls of microorganisms, effectively killing them and preventing their replication.
Can hypochlorous acid be used in food processing?
Yes, hypochlorous acid is used in food processing to disinfect surfaces and equipment, ensuring food safety and reducing the risk of foodborne illnesses.
What are the environmental benefits of using hypochlorous acid?
Hypochlorous acid is environmentally friendly due to its non-toxic and biodegradable nature. It breaks down into harmless by-products, such as water and salt, after use, minimizing its environmental impact.
Is hypochlorous acid effective against antibiotic-resistant bacteria?
Yes, hypochlorous acid is effective against a wide range of pathogens, including antibiotic-resistant bacteria, thanks to its powerful oxidizing properties that disrupt cell walls.
Conclusion
In conclusion, hypochlorous acid is a remarkable compound with a wide range of applications in various industries. Its simple chemical formula, HOCl, belies its potent antimicrobial properties and effectiveness as a disinfectant. From healthcare to food safety and water treatment, hypochlorous acid plays a crucial role in maintaining hygiene and reducing the risk of infections.
Its non-toxic and environmentally friendly nature makes it an ideal choice for industries looking to enhance safety and hygiene standards without compromising the environment. As research continues to explore its potential, hypochlorous acid is set to become an even more integral part of our daily lives, offering safe and effective solutions to modern challenges.
Overall, the hypochlorous acid formula is a testament to the power of chemistry in improving our world, providing valuable insights into the potential of simple compounds to make a significant impact on health, safety, and sustainability.
For more detailed information on the applications and benefits of hypochlorous acid, consider visiting reputable sources such as the Centers for Disease Control and Prevention (CDC) or the World Health Organization (WHO).