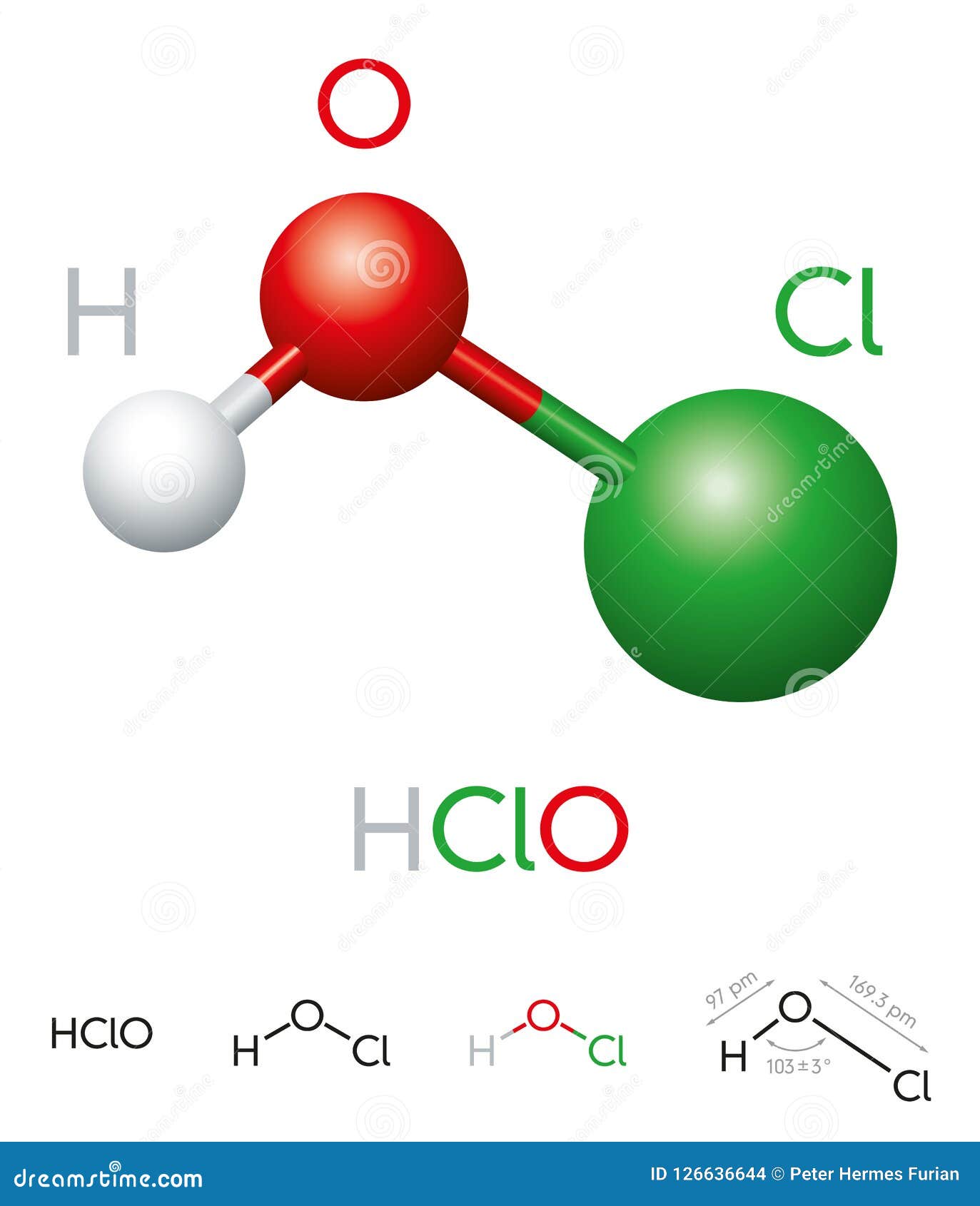
Hypochlorous acid, a remarkable and versatile chemical, has gained significant attention in various fields due to its unique properties and applications. The chemical formula of hypochlorous acid, HOCl, may seem simple, but its impact is profound. From its role in disinfection to its potential uses in medicine, hypochlorous acid continues to be a topic of interest for researchers and industry professionals alike.
Understanding the chemistry behind hypochlorous acid is crucial for appreciating its functions. The molecule consists of one hydrogen atom, one oxygen atom, and one chlorine atom. Despite its simplicity, it is an effective antimicrobial agent, which makes it invaluable in healthcare and sanitation. Its ability to eliminate bacteria, viruses, and fungi without harming human tissues makes it an ideal choice for various applications.
In recent years, the interest in hypochlorous acid has surged as people seek safer and more environmentally friendly alternatives to traditional disinfectants. This article will delve into the hypochlorous acid chemical formula, explore its diverse applications, and provide insights into its benefits and limitations. Whether you're a chemistry enthusiast or a professional in the field, this comprehensive guide will equip you with the knowledge you need to harness the power of hypochlorous acid effectively.
Read also:Everything You Need To Know About 5moviez Rulz The Ultimate Entertainment Hub
Table of Contents
- The Chemistry of Hypochlorous Acid
- How Does Hypochlorous Acid Work?
- Applications in Disinfection
- Role in Medicine and Healthcare
- Environmental Impact and Benefits
- Production Methods of Hypochlorous Acid
- Safety Considerations
- What Are the Limitations?
- Future Prospects and Research
- How Can It Be Used at Home?
- Hypochlorous Acid vs Other Disinfectants
- Economic Aspects
- Frequently Asked Questions
- Conclusion
The Chemistry of Hypochlorous Acid
Hypochlorous acid (HOCl) is a weak acid that forms when chlorine dissolves in water. It is a member of the halogen oxoacids family and is an intermediate species in the chlorine disinfection process. The chemical structure of hypochlorous acid is defined by its molecular composition: one hydrogen atom, one oxygen atom, and one chlorine atom. This simple molecular structure is responsible for its unique properties.
The formation of hypochlorous acid in aqueous solutions occurs through a reversible reaction between chlorine and water. The equation is as follows:
- Cl2 + H2O ↔ HOCl + HCl
Hypochlorous acid is a strong oxidizing agent, which makes it highly effective in breaking down organic materials and killing microorganisms. Its antimicrobial properties are due to its ability to penetrate cell walls, disrupting cellular processes and leading to cell death. This makes HOCl a powerful disinfectant with broad-spectrum antimicrobial activity.
How Does Hypochlorous Acid Work?
The mode of action of hypochlorous acid as a disinfectant is largely attributed to its ability to disrupt microbial cell membranes and inactivate vital cellular functions. Once hypochlorous acid penetrates the cell wall, it reacts with various cellular components, including proteins, lipids, and nucleic acids. This leads to oxidative damage and denaturation of essential biomolecules, effectively neutralizing the microorganism.
Some key points about the mechanism of action include:
- Disruption of cell membrane integrity
- Oxidation of sulfhydryl groups in proteins
- Inactivation of enzyme systems
- Denaturation of nucleic acids
These actions collectively contribute to the antimicrobial efficacy of hypochlorous acid, making it a preferred choice in many disinfection protocols. Its effectiveness against a wide range of pathogens, including bacteria, viruses, and fungi, highlights its versatility as a disinfectant.
Read also:Ultimate Guide To Hd Movie Hub 4u Your Gateway To Highquality Entertainment
Applications in Disinfection
Hypochlorous acid has gained prominence as a disinfectant in various settings due to its efficacy and safety profile. It is used extensively in healthcare, food processing, agriculture, and public sanitation. The ability to produce hypochlorous acid on-site through electrolysis of saltwater is another advantage, as it ensures a fresh and potent supply of the disinfectant.
Role in Medicine and Healthcare
In medicine and healthcare, hypochlorous acid is used for wound care, surgical site disinfection, and as a hand sanitizer alternative. Its non-toxic nature allows for safe use on skin and mucous membranes, reducing the risk of irritation and allergic reactions.
Environmental Impact and Benefits
One of the significant benefits of hypochlorous acid is its minimal environmental impact. Unlike many traditional disinfectants, it breaks down into harmless substances, such as water and salt, after use. This makes it an environmentally friendly option for large-scale disinfection operations, reducing the ecological footprint of sanitation efforts.
Production Methods of Hypochlorous Acid
Hypochlorous acid can be produced through various methods, including chemical synthesis and electrolysis. The most common method is the electrolysis of saltwater, which involves passing an electric current through a saline solution to produce hypochlorous acid along with sodium hydroxide and hydrogen gas. This method is favored for its simplicity and ability to produce hypochlorous acid on-site, ensuring freshness and potency.
Safety Considerations
While hypochlorous acid is generally considered safe for use, it is essential to adhere to safety guidelines to prevent any potential risks. Proper handling and storage of hypochlorous acid solutions are crucial to maintain their stability and effectiveness. Additionally, users should be aware of the concentration levels and ensure that they do not exceed recommended limits, as high concentrations can cause irritation or damage to tissues.
What Are the Limitations?
Despite its many advantages, hypochlorous acid has limitations that users should consider. It can be unstable over time, especially when exposed to light and heat, which may reduce its effectiveness. Additionally, its efficacy can be affected by the presence of organic matter, requiring thorough cleaning of surfaces before application. Understanding these limitations is crucial for optimizing the use of hypochlorous acid in various applications.
Future Prospects and Research
As interest in hypochlorous acid continues to grow, ongoing research aims to explore new applications and improve existing formulations. Innovations in production methods and stabilization techniques are likely to enhance its effectiveness and expand its use in various industries. The potential of hypochlorous acid in emerging fields such as biotechnology and environmental science presents exciting opportunities for future advancements.
How Can It Be Used at Home?
Hypochlorous acid solutions can be used in domestic settings for cleaning and disinfection purposes. Homeowners can utilize hypochlorous acid for sanitizing kitchen surfaces, bathrooms, and even fresh produce. Its non-toxic nature makes it safe for use around children and pets, providing peace of mind for families seeking effective cleaning solutions.
Hypochlorous Acid vs Other Disinfectants
When compared to other disinfectants, hypochlorous acid stands out for its safety, effectiveness, and environmental benefits. Unlike chlorine bleach or alcohol-based sanitizers, hypochlorous acid does not produce harmful fumes or residues, making it a safer option for indoor use. Additionally, its rapid action and broad-spectrum efficacy make it a competitive choice in the disinfection landscape.
Economic Aspects
The economic considerations of using hypochlorous acid involve analyzing the cost-effectiveness of production and application. While the initial setup for on-site production may require investment, the long-term savings from reduced chemical purchases and waste management can be significant. Furthermore, the shift towards sustainable and environmentally friendly disinfectants aligns with global trends, potentially driving demand and market growth.
Frequently Asked Questions
- What is the hypochlorous acid chemical formula?
The chemical formula for hypochlorous acid is HOCl.
- Is hypochlorous acid safe for human use?
Yes, hypochlorous acid is safe for human use when used at appropriate concentrations. It is often used for wound care and as a disinfectant without causing irritation.
- How effective is hypochlorous acid against viruses?
Hypochlorous acid is highly effective against a wide range of viruses, including coronaviruses, due to its ability to disrupt viral envelopes and inactivate viral particles.
- Can hypochlorous acid be used on food surfaces?
Yes, hypochlorous acid is commonly used to sanitize food surfaces and fresh produce, as it does not leave harmful residues.
- How long does hypochlorous acid remain effective after production?
Hypochlorous acid can remain effective for several days if stored properly in a cool, dark place. However, its stability decreases over time, especially when exposed to light and heat.
- What are the environmental benefits of using hypochlorous acid?
Hypochlorous acid is environmentally friendly because it breaks down into water and salt, leaving no harmful residues or by-products.
Conclusion
Hypochlorous acid, with its simple yet potent chemical formula, offers a wealth of applications across various industries. Its effectiveness as a disinfectant, coupled with its safety and environmental benefits, makes it an invaluable tool in modern sanitation practices. As research continues to unlock new potential uses and improve production methods, hypochlorous acid is poised to play an increasingly important role in promoting public health and environmental sustainability. By understanding its properties and applications, individuals and businesses can harness the power of hypochlorous acid to address contemporary disinfection challenges effectively.