The world of chemistry introduces us to a myriad of compounds, each with unique properties and applications. Among these, hypochlorous acid stands out due to its versatility and effectiveness in various domains. Known for its disinfectant qualities, hypochlorous acid plays a vital role in numerous industries, including healthcare, sanitation, and agriculture. Understanding the formula for hypochlorous acid is crucial for those interested in chemistry or seeking to harness its potential benefits.
Hypochlorous acid, often represented by the chemical formula HOCl, is a weak acid formed when chlorine dissolves in water. This compound is renowned for its potent antimicrobial properties, making it a popular choice in sanitization and cleaning products. The simplicity of its formula belies its powerful capabilities, as it effectively neutralizes bacteria, viruses, and other pathogens upon contact.
In recent years, the importance of hypochlorous acid has grown, particularly in the context of public health and safety. With a growing emphasis on hygiene and cleanliness, understanding how to utilize hypochlorous acid effectively is more relevant than ever. This article delves into the details of hypochlorous acid, exploring its chemical structure, applications, and benefits, all while keeping the formula for hypochlorous acid at the forefront of the discussion.
Read also:Movierulzla Kannada 2024 A Deep Dive Into The World Of Kannada Cinema And Online Streaming
Table of Contents
- What is Hypochlorous Acid?
- Chemical Structure of Hypochlorous Acid
- How is Hypochlorous Acid Formed?
- Properties of Hypochlorous Acid
- Applications of Hypochlorous Acid
- Role in Disinfection and Sanitization
- Use in Healthcare and Medicine
- Agricultural Applications
- Industrial and Commercial Uses
- Safety Considerations
- Is Hypochlorous Acid Environmentally Friendly?
- Advantages and Disadvantages of Hypochlorous Acid
- Frequently Asked Questions
- Conclusion
What is Hypochlorous Acid?
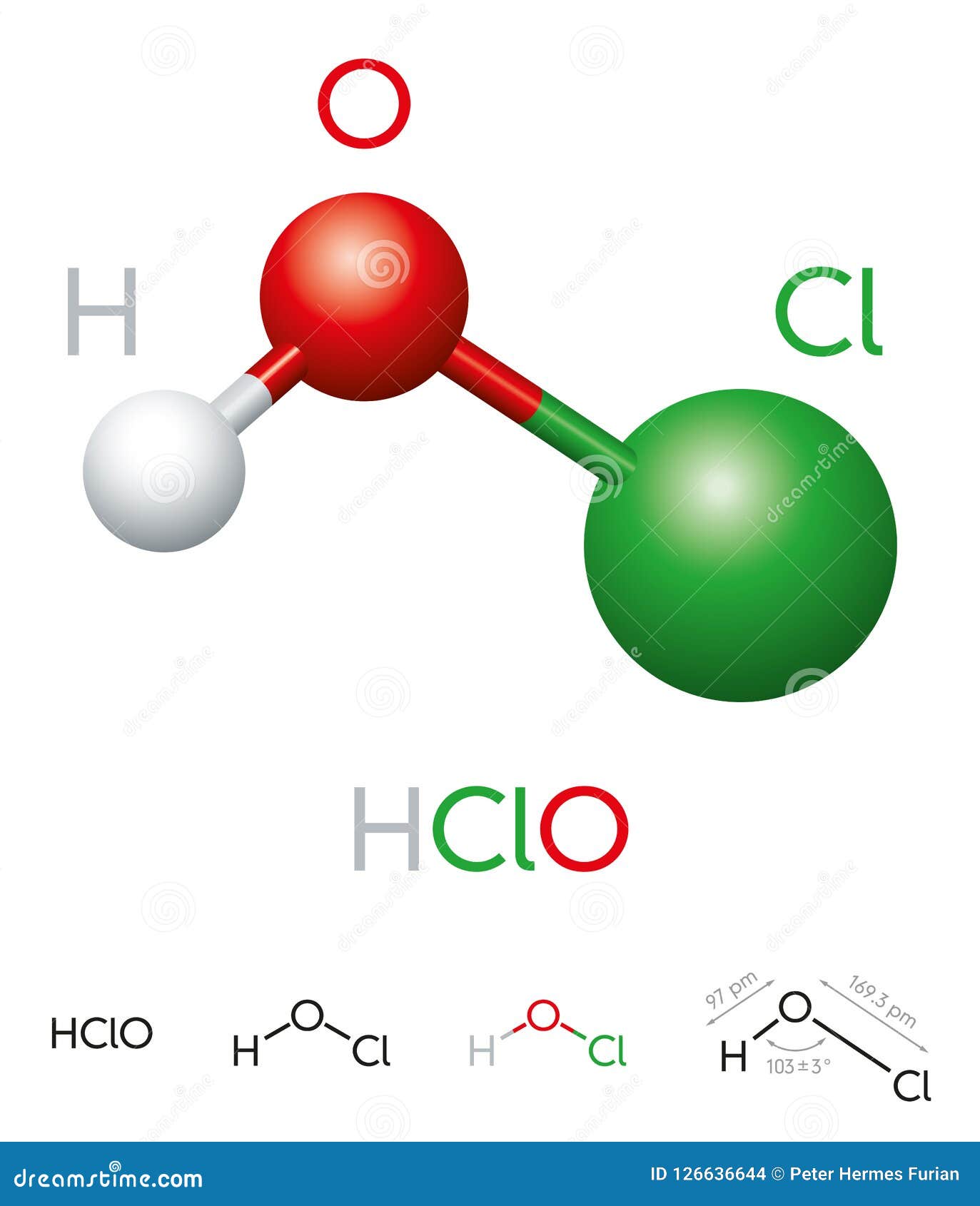
Hypochlorous acid is a weak acid with the chemical formula HOCl. It is formed when chlorine dissolves in water, establishing equilibrium with its precursor, chlorine gas (Cl2). Despite being a weak acid, hypochlorous acid is highly effective as an antimicrobial agent, making it widely used in disinfectants and sanitizers.
Chemical Structure of Hypochlorous Acid
The chemical structure of hypochlorous acid is relatively simple, consisting of one hydrogen atom, one oxygen atom, and one chlorine atom. The molecular structure can be represented as HOCl, where the atoms are bonded covalently. This simplicity contributes to its effectiveness in disrupting microbial cell walls, leading to their destruction.
How is Hypochlorous Acid Formed?
Hypochlorous acid is formed through the dissolution of chlorine in water. When chlorine gas is introduced to water, it reacts to form hypochlorous acid and hydrochloric acid (HCl) as per the following reaction:
Cl2 + H2O ⇌ HOCl + HCl
This reaction is reversible, and the equilibrium can shift based on factors such as pH and temperature. In aqueous solutions, hypochlorous acid exists in equilibrium with its ionic form, hypochlorite (OCl-), particularly in alkaline conditions.
Properties of Hypochlorous Acid
Hypochlorous acid exhibits several key properties that make it effective as a disinfectant and sanitizer:
Read also:Ultimate Guide To 7 Movierulz 2024 Download Everything You Need To Know
- Oxidizing Agent: HOCl acts as a powerful oxidizing agent, disrupting microbial cell walls and leading to the destruction of pathogens.
- Antimicrobial Efficacy: It is effective against a wide range of microorganisms, including bacteria, viruses, and fungi.
- Non-Toxic: In appropriate concentrations, hypochlorous acid is safe for human exposure and does not leave harmful residues.
- pH Sensitivity: Its effectiveness is influenced by pH, with optimal activity observed at slightly acidic to neutral pH levels.
Applications of Hypochlorous Acid
The applications of hypochlorous acid are vast, ranging from household cleaning to industrial processes. Its versatility and safety make it a preferred choice in various sectors.
Role in Disinfection and Sanitization
Hypochlorous acid is widely used in disinfection and sanitization efforts, particularly in healthcare and hospitality industries. Its ability to effectively eliminate pathogens without leaving toxic residues makes it an ideal choice for maintaining hygiene and safety.
Use in Healthcare and Medicine
In healthcare settings, hypochlorous acid is used for wound care, surgical site disinfection, and sanitation of medical equipment. Its non-toxic nature ensures that it does not harm patients or healthcare workers, while effectively reducing the risk of infections.
Agricultural Applications
Hypochlorous acid is gaining traction in agriculture, where it is used to sanitize produce and equipment, ensuring food safety and reducing the risk of contamination. It is also employed in water treatment for irrigation systems, promoting healthy crop growth.
Industrial and Commercial Uses
Industrially, hypochlorous acid is utilized in water treatment facilities, food processing plants, and textile industries. Its ability to disinfect and sanitize effectively while being environmentally friendly makes it a valuable asset in these sectors.
Safety Considerations
While hypochlorous acid is considered safe for use in various applications, certain precautions are necessary to ensure its effective and safe usage:
- Concentration Levels: Ensure appropriate concentration levels to avoid irritation or adverse effects.
- Storage: Store in a cool, dark place to maintain stability and efficacy.
- Handling: Use protective gear when handling concentrated solutions to prevent skin and eye irritation.
Is Hypochlorous Acid Environmentally Friendly?
Yes, hypochlorous acid is considered environmentally friendly. It breaks down into non-toxic byproducts, does not accumulate in the environment, and poses minimal risk to aquatic life. Its use in various sectors aligns with sustainable practices and eco-friendly initiatives.
Advantages and Disadvantages of Hypochlorous Acid
Hypochlorous acid offers several advantages, but it is essential to consider its limitations as well:
Advantages:
- Broad-spectrum antimicrobial activity
- Non-toxic and non-irritating at appropriate concentrations
- Environmentally friendly with minimal impact
- Easy to produce and use
Disadvantages:
- pH sensitivity affecting efficacy
- Requires proper storage and handling
- Limited shelf life and stability
Frequently Asked Questions
What is the chemical formula for hypochlorous acid?
The chemical formula for hypochlorous acid is HOCl.
How does hypochlorous acid kill bacteria?
Hypochlorous acid kills bacteria by oxidizing their cell walls, leading to the disruption and destruction of the cells.
Can hypochlorous acid be used for wound care?
Yes, hypochlorous acid is used in wound care due to its non-toxic nature and ability to reduce infection risk without harming healthy tissue.
Is hypochlorous acid safe for use on food surfaces?
Yes, hypochlorous acid is safe for use on food surfaces and is often used to sanitize produce and equipment in the food industry.
What is the optimal pH level for hypochlorous acid activity?
The optimal pH level for hypochlorous acid activity is slightly acidic to neutral, typically between pH 5 to 7.
Are there alternatives to hypochlorous acid for disinfection?
Yes, alternatives include hydrogen peroxide, alcohol-based sanitizers, and quaternary ammonium compounds, though each has its own advantages and limitations.
Conclusion
Understanding the formula for hypochlorous acid and its applications enhances our ability to utilize this powerful compound effectively. As a versatile and environmentally friendly disinfectant, hypochlorous acid continues to play a critical role in public health, agriculture, and industry. By leveraging its properties and advantages, we can continue to promote safety and hygiene across various sectors.