In the world of chemistry, understanding the formula of hypochlorous acid is crucial not only for students and researchers but also for professionals in various industries. A compound that might seem obscure at first glance, hypochlorous acid plays a significant role in disinfection, sanitation, and even within our own immune systems. Its chemical formula, HOCl, provides insight into its composition and reactivity. With its applications ranging from household cleaning agents to medical sterilization, hypochlorous acid has become an integral part of modern life.
Hypochlorous acid, with its simple molecular structure, invites curiosity about its properties and uses. Comprising hydrogen, oxygen, and chlorine, this compound's formula is deceptively straightforward. However, the implications of its use and the chemistry behind its reactions are profound. As a weak acid, it dissociates in water to form ions that are effective against a wide range of pathogens. This makes it particularly valuable in settings where hygiene and safety are paramount.
The significance of hypochlorous acid extends beyond its chemical formula. It has found a place in history as a powerful disinfectant, being utilized in various forms since its discovery. Today, its applications are diverse, ranging from water purification to wound care. By delving into the specifics of its chemical makeup, we can appreciate not only its effectiveness but also the science that allows us to harness its benefits safely and efficiently.
Read also:Movierulzcom Kannada Movies Download Guide Everything You Need To Know
Table of Contents
- What is Hypochlorous Acid?
- The Chemical Formula: HOCl
- How is Hypochlorous Acid Produced?
- Why is Hypochlorous Acid Effective as a Disinfectant?
- Applications in Daily Life
- Industrial and Medical Uses
- The Role of Hypochlorous Acid in the Human Body
- Environmental Impact of Hypochlorous Acid
- Safety Considerations and Handling
- Comparative Analysis: Hypochlorous Acid vs. Other Disinfectants
- FAQs about Hypochlorous Acid
- Conclusion
What is Hypochlorous Acid?
Hypochlorous acid is a weak, unstable acid with the chemical formula HOCl. It is known for its bleaching, disinfecting, and oxidizing properties. This compound is commonly encountered in diluted forms, such as in bleach solutions, where it serves as the active antimicrobial agent. Hypochlorous acid is also naturally produced by white blood cells in the human body as part of the immune response to fight off pathogens.
Given its volatile nature, hypochlorous acid cannot be stored for long periods and is often generated on-site where needed. This characteristic makes it ideal for applications requiring immediate and effective disinfection. The acid's reactivity stems from its ability to release chlorine, which is highly effective in neutralizing bacteria and viruses. As a result, it is widely used in various sectors, including healthcare, food processing, and water treatment.
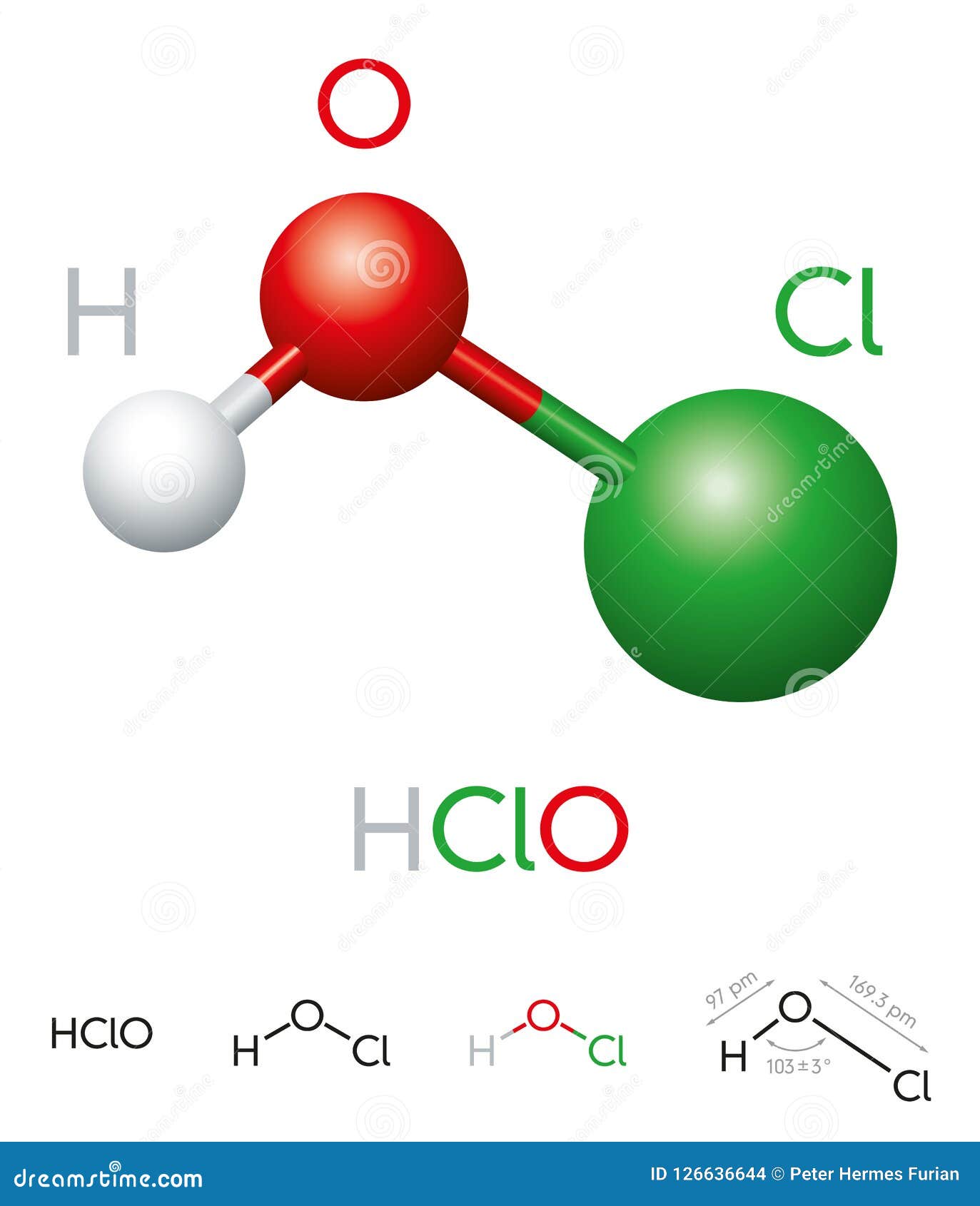
The Chemical Formula: HOCl
The chemical formula of hypochlorous acid, HOCl, reveals its composition: one hydrogen (H) atom, one oxygen (O) atom, and one chlorine (Cl) atom. This simple arrangement belies the compound's powerful chemical activity. The structure of HOCl is such that it allows the release of chlorine, which acts as the primary disinfectant. This makes it highly effective for sanitation purposes.
The formation of hypochlorous acid occurs when chlorine is dissolved in water, undergoing a reaction that produces both hypochlorous acid and hydrochloric acid. The balance of these acids in solution depends on the pH level, with lower pH favoring the presence of hypochlorous acid. This characteristic is crucial to its use in various applications, as the effectiveness as a disinfectant is pH-dependent.
How is Hypochlorous Acid Produced?
Hypochlorous acid is typically produced through the reaction of chlorine gas with water. This process can be represented by the chemical equation: Cl2 + H2O → HOCl + HCl. In this reaction, chlorine gas (Cl2) is bubbled into water, resulting in the formation of hypochlorous acid (HOCl) and hydrochloric acid (HCl).
Another method of producing hypochlorous acid involves the electrolysis of saltwater. This method is particularly useful in generating hypochlorous acid on-site for industrial and commercial purposes. The process involves passing an electric current through a saline solution, which results in the formation of chlorine gas, hydrogen gas, and sodium hydroxide. The chlorine gas then reacts with water to form hypochlorous acid.
Read also:Essential Guide To The 5movie Rules For Film Enthusiasts
Why is Hypochlorous Acid Effective as a Disinfectant?
The effectiveness of hypochlorous acid as a disinfectant is primarily due to its ability to penetrate cell walls and disrupt cellular functions of microorganisms. Its neutral charge allows it to easily pass through the protective barriers of bacteria and viruses, leading to their rapid inactivation. This makes hypochlorous acid particularly effective against a broad spectrum of pathogens, including bacteria, viruses, and fungi.
Additionally, hypochlorous acid is highly reactive, which enables it to destroy a wide range of microbial cells. Unlike other disinfectants that may only target specific types of microorganisms, hypochlorous acid's mode of action allows it to be universally effective. This broad-spectrum efficacy is one of the reasons it is widely used in environments where hygiene is critical, such as hospitals, food processing facilities, and public water systems.
Applications in Daily Life
In everyday life, hypochlorous acid is most commonly encountered in cleaning and disinfecting products. It is an active ingredient in many household bleach and cleaning solutions, where it serves to sanitize surfaces and eliminate harmful microorganisms. Its effectiveness at low concentrations makes it safe for use in a variety of settings, from home kitchens to schools and offices.
Besides cleaning, hypochlorous acid is also used in personal hygiene products, such as hand sanitizers and wound care solutions. Its gentle yet effective action makes it suitable for use on skin, where it can help prevent infections and promote healing. The versatility of hypochlorous acid in these applications highlights its significant role in maintaining public health and safety.
Industrial and Medical Uses
In industrial settings, hypochlorous acid is used extensively for its disinfecting properties. It is applied in the food industry to sanitize equipment and surfaces, ensuring products are free from contamination. In the medical field, hypochlorous acid is used in wound care and as a sterilizing agent for medical instruments. Its ability to kill a wide range of pathogens without causing harm to human tissues makes it invaluable in healthcare environments.
Moreover, hypochlorous acid is employed in water treatment processes to purify drinking water and ensure it is safe for consumption. Its use in municipal water systems helps control waterborne diseases, protecting public health on a large scale. The adaptability of hypochlorous acid across these varied applications underscores its importance in both industrial and medical contexts.
The Role of Hypochlorous Acid in the Human Body
Hypochlorous acid plays a crucial role in the human body's immune system, where it is produced by white blood cells known as neutrophils. When the body detects an invading pathogen, neutrophils release hypochlorous acid to neutralize the threat. This action is part of the body's natural defense mechanism, highlighting hypochlorous acid's importance in maintaining health and preventing infections.
The production of hypochlorous acid in the body is highly controlled to ensure it effectively targets pathogens without damaging healthy cells. This balance is essential for the immune system to function properly and protect the body from disease. Understanding this natural process provides insights into how hypochlorous acid can be used safely and effectively in medical treatments and hygiene products.
Environmental Impact of Hypochlorous Acid
As a disinfectant, hypochlorous acid is considered environmentally friendly due to its rapid degradation into harmless byproducts. Unlike some chemical disinfectants that persist in the environment and cause pollution, hypochlorous acid breaks down into water and salt, minimizing its ecological footprint. This property makes it a preferred choice for sustainable practices in industries concerned with environmental impact.
However, like any chemical, the use of hypochlorous acid must be managed appropriately to prevent potential negative effects. Excessive use or improper disposal can lead to localized chlorine pollution, which can harm aquatic life. Therefore, it is important for industries and individuals alike to adhere to guidelines and regulations regarding the use and disposal of hypochlorous acid.
Safety Considerations and Handling
When handling hypochlorous acid, safety is paramount due to its reactive nature. Although it is less corrosive than other disinfectants, appropriate precautions should still be taken to prevent irritation or injury. Personal protective equipment, such as gloves and goggles, should be worn when handling concentrated solutions to avoid skin and eye contact.
Storage of hypochlorous acid requires consideration of its instability. It should be kept in a cool, dark place, away from direct sunlight and heat sources. Containers should be sealed tightly to prevent the release of chlorine gas, which can be hazardous. By following these guidelines, hypochlorous acid can be used safely and effectively in a variety of applications.
Comparative Analysis: Hypochlorous Acid vs. Other Disinfectants
When compared to other disinfectants, hypochlorous acid offers several advantages. Its rapid action and broad-spectrum efficacy make it more effective than many alternatives. Unlike alcohol-based disinfectants, it does not evaporate quickly, allowing for prolonged contact time with surfaces and ensuring thorough disinfection.
Additionally, hypochlorous acid is less likely to cause irritation or allergic reactions, making it a safer option for individuals with sensitive skin or respiratory conditions. Its environmental profile is also more favorable compared to chemical disinfectants that leave harmful residues. These factors contribute to its growing popularity as a preferred disinfectant in various settings.
FAQs about Hypochlorous Acid
1. What is the formula of hypochlorous acid?
The formula of hypochlorous acid is HOCl.
2. How is hypochlorous acid used in healthcare?
Hypochlorous acid is used in healthcare for wound care, sterilization of medical instruments, and as a disinfectant in hospitals and clinics.
3. Is hypochlorous acid safe for use on skin?
Yes, hypochlorous acid is safe for use on skin and is often included in wound care products and sanitizers due to its gentle yet effective antimicrobial properties.
4. Can hypochlorous acid be used in food processing?
Yes, hypochlorous acid is used in the food industry to sanitize equipment and surfaces, helping to ensure food safety and prevent contamination.
5. How does hypochlorous acid compare to bleach?
Hypochlorous acid is more effective at lower concentrations than bleach and is less corrosive, making it a safer option for many applications.
6. What are the environmental implications of using hypochlorous acid?
Hypochlorous acid is environmentally friendly due to its rapid degradation into harmless byproducts, but proper use and disposal are necessary to prevent localized pollution.
Conclusion
Understanding the formula of hypochlorous acid and its wide-ranging applications reveals its importance in modern society. From its role in the immune system to its use as a versatile disinfectant, hypochlorous acid continues to be an essential compound in both everyday life and industrial processes. As we continue to prioritize health and environmental sustainability, the safe and effective use of hypochlorous acid will remain a critical component in achieving these goals.
For more detailed information on the uses and safety guidelines of hypochlorous acid, you can visit CDC's official website.