Hypochlorous acid, a compound of significant importance in various industries, is often recognized for its role in sanitation and disinfection. Its chemical formula, a simple yet profound representation, is HOCl. This formula unveils the compound's composition, revealing a blend of hydrogen, oxygen, and chlorine, elements that contribute to its unique properties and applications. As we delve deeper into understanding hypochlorous acid, this article will explore its chemical structure, uses, and benefits.
In the realm of chemistry, hypochlorous acid stands out for its potent antimicrobial properties. Its chemical formula, HOCl, is central to its effectiveness, particularly in the healthcare and food industries. As a weak acid, it is known for being a powerful oxidizing agent, making it invaluable in environments that demand stringent hygiene standards. Understanding its formula not only aids in appreciating its applications but also underscores its role in maintaining health and safety across various sectors.
This article aims to provide a thorough exploration of hypochlorous acid, focusing on its chemical formula and the implications of this composition. By examining its structure, we can gain insights into how this acid functions and why it is an essential component in many disinfection processes. Whether you're a student, a professional in the field, or simply curious about the science behind everyday compounds, this guide will offer valuable knowledge about hypochlorous acid and its relevance in our world.
Read also:Ultimate Guide To 5movierulzpe Everything You Need To Know
Table of Contents
- History and Origin of Hypochlorous Acid
- Chemical Composition and Structure
- How Does Hypochlorous Acid Work?
- Applications of Hypochlorous Acid
- Benefits and Advantages of Hypochlorous Acid
- Hypochlorous Acid in Healthcare
- Environmental Impact of Hypochlorous Acid
- Is Hypochlorous Acid Safe?
- Production Methods of Hypochlorous Acid
- Comparison with Other Disinfectants
- Storage and Stability Considerations
- Chemical Reactions Involving Hypochlorous Acid
- Future Prospects of Hypochlorous Acid
- Frequently Asked Questions
- Conclusion
History and Origin of Hypochlorous Acid
The history of hypochlorous acid dates back to the early 19th century, when it was first discovered by French chemist Antoine Jérôme Balard in 1834. Balard's work primarily focused on the decomposition of chlorine in water, leading to the identification of hypochlorous acid. This discovery marked a significant milestone in the field of chemistry, as it paved the way for understanding chlorine's behavior in aqueous solutions.
Initially, hypochlorous acid was not fully appreciated for its potential applications. It wasn't until the late 19th and early 20th centuries that its antimicrobial properties were recognized, sparking interest in its use as a disinfectant. The development of industrial processes for producing hypochlorous acid further facilitated its adoption in various sectors, particularly in public health and sanitation.
Today, hypochlorous acid is widely used across the globe, thanks to its effectiveness in killing bacteria and viruses. Its journey from a mere chemical curiosity to a vital component in disinfection demonstrates the evolving understanding of chemical compounds and their applications in improving human health and safety.
Chemical Composition and Structure
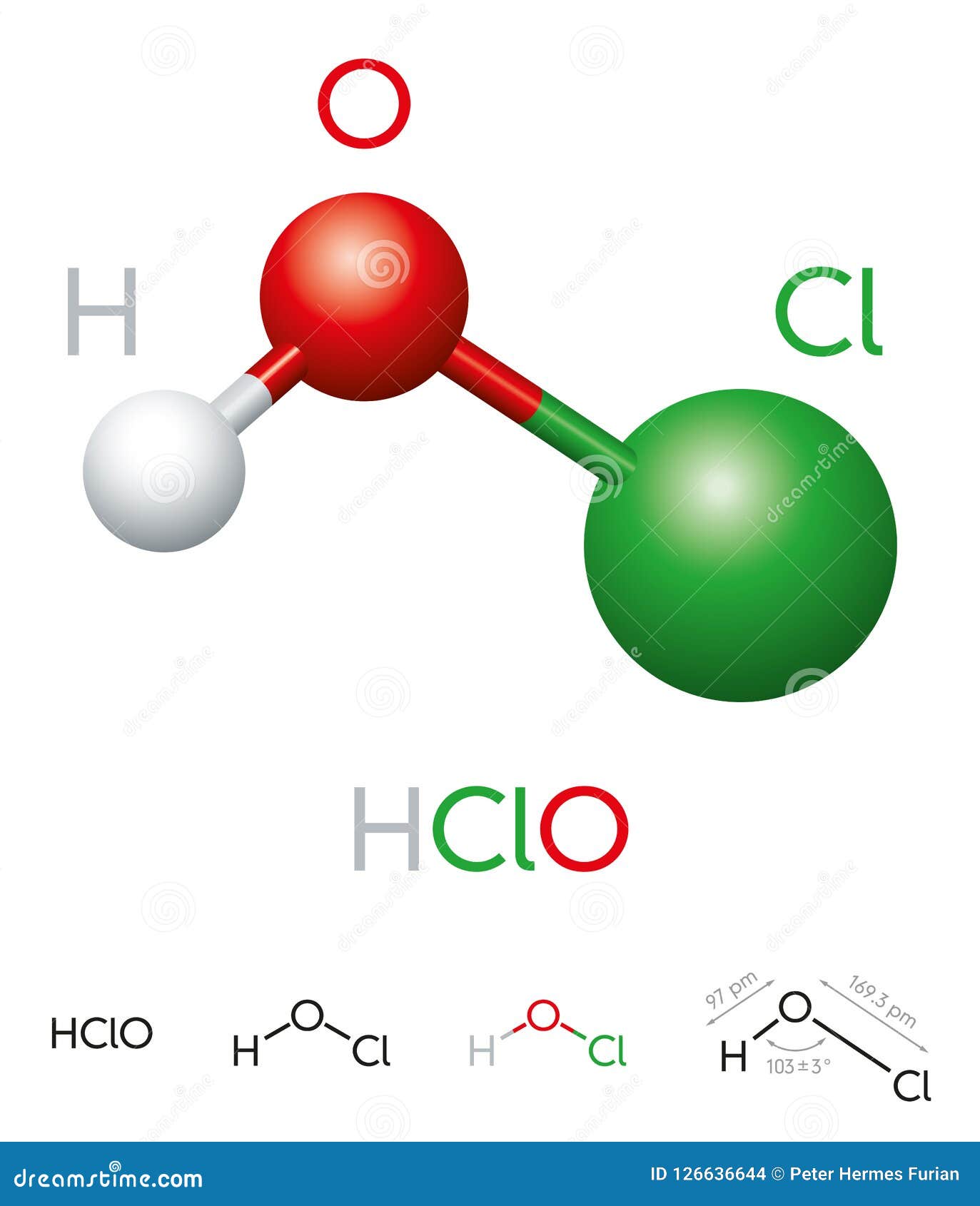
The chemical formula for hypochlorous acid is HOCl, representing the combination of hydrogen (H), oxygen (O), and chlorine (Cl). This simple yet powerful formula reflects the acid's unique properties and effectiveness as a disinfectant. The structure of hypochlorous acid consists of a single hydrogen atom bonded to an oxygen atom, which is in turn bonded to a chlorine atom.
The molecular geometry of hypochlorous acid is bent, with the oxygen atom serving as the central atom. This bent structure is crucial to its reactivity, particularly in its ability to oxidize and destroy microbial cells. The presence of chlorine, a highly electronegative element, enhances the acid's ability to break down organic matter and neutralize harmful pathogens.
Understanding the chemical composition of hypochlorous acid is key to appreciating its efficacy as a disinfectant. The presence of both oxidizing and antimicrobial properties makes it a versatile compound, capable of addressing a wide range of sanitation needs in diverse environments.
Read also:Julie Neal Pitt A Life Of Purpose And Inspiration
How Does Hypochlorous Acid Work?
Hypochlorous acid functions primarily as an oxidizing agent, targeting and disrupting the cell walls of bacteria, viruses, and other pathogens. Upon contact with these microorganisms, hypochlorous acid penetrates the cell membrane, leading to the denaturation of proteins and the destruction of essential cellular components.
One of the key mechanisms behind hypochlorous acid's effectiveness is its ability to produce reactive oxygen species (ROS). These highly reactive molecules interact with the lipids, proteins, and nucleic acids within microbial cells, causing oxidative damage that ultimately results in cell death. This process is particularly effective against a wide range of bacteria, including antibiotic-resistant strains, as well as viruses and fungi.
The ability of hypochlorous acid to rapidly neutralize pathogens makes it an invaluable tool in environments where hygiene is paramount. Its non-toxic nature and minimal residue make it suitable for use in healthcare settings, food processing, and water treatment, among other applications.
Applications of Hypochlorous Acid
The versatility of hypochlorous acid is evident in its wide range of applications across various industries. In healthcare, it is used for sanitation and infection control, particularly in hospitals and clinics where preventing the spread of infectious diseases is critical. Hypochlorous acid's ability to kill bacteria and viruses without causing harm to human tissues makes it an ideal choice for wound care and skin disinfection.
In the food industry, hypochlorous acid is employed as a sanitizer to ensure food safety and quality. It is used to disinfect surfaces, equipment, and even food products themselves, reducing the risk of contamination and spoilage. Its efficacy in eliminating pathogens without altering the taste or nutritional value of food makes it a preferred option for food processing and preparation.
Additionally, hypochlorous acid is used in water treatment processes to purify drinking water and maintain water quality in swimming pools and spas. Its ability to remove organic contaminants and control microbial growth helps ensure safe and clean water for consumption and recreational use.
Benefits and Advantages of Hypochlorous Acid
Hypochlorous acid offers several benefits and advantages over traditional disinfectants. One of its most significant advantages is its safety profile. Unlike many chemical disinfectants, hypochlorous acid is non-toxic and non-irritating, making it safe for use around humans, animals, and the environment. Its gentle nature allows for direct application on skin and surfaces without causing harm or damage.
Another benefit of hypochlorous acid is its rapid action. It works quickly to neutralize a broad spectrum of pathogens, providing effective sanitation in a short amount of time. This rapid action is particularly beneficial in settings where time is of the essence, such as in healthcare facilities and food processing plants.
Hypochlorous acid is also environmentally friendly, breaking down into harmless byproducts that do not contribute to pollution or environmental degradation. Its biodegradability makes it an attractive option for sustainable sanitation practices, aligning with the growing emphasis on eco-friendly solutions in various industries.
Hypochlorous Acid in Healthcare
The role of hypochlorous acid in healthcare is multifaceted, with its primary application being in infection control and prevention. Hospitals and clinics utilize hypochlorous acid for surface disinfection, sterilization of medical equipment, and even hand hygiene, thanks to its potent antimicrobial properties and safety for human contact.
In wound care, hypochlorous acid is used to clean and disinfect wounds, promoting healing while minimizing the risk of infection. Its ability to kill bacteria without damaging healthy tissue makes it a valuable tool in treating chronic wounds, burns, and surgical sites. Additionally, hypochlorous acid is employed in dermatology for treating skin infections and conditions such as acne, due to its anti-inflammatory and antimicrobial effects.
The adoption of hypochlorous acid in healthcare settings continues to grow, driven by its effectiveness, safety, and adaptability to various applications. As concerns about antibiotic resistance and healthcare-associated infections rise, hypochlorous acid presents a viable solution for maintaining hygiene and protecting patient health.
Environmental Impact of Hypochlorous Acid
Hypochlorous acid is recognized for its minimal environmental impact, making it an environmentally friendly choice for disinfection and sanitation. Upon application, hypochlorous acid breaks down into water and chloride ions, both of which are harmless to the environment. This degradation process ensures that no toxic residues are left behind, reducing the risk of pollution and environmental harm.
Furthermore, the production and use of hypochlorous acid align with sustainable practices. It can be generated on-site using simple electrolysis of saltwater, minimizing the need for transportation and reducing the carbon footprint associated with distribution. This method of production not only lowers environmental impact but also provides an efficient and cost-effective solution for sanitation needs.
The eco-friendly nature of hypochlorous acid is increasingly important in a world where environmental conservation is a priority. Industries and organizations seeking to implement sustainable practices are turning to hypochlorous acid as a reliable and responsible choice for maintaining hygiene and safety without compromising environmental integrity.
Is Hypochlorous Acid Safe?
The safety of hypochlorous acid is well-documented, with numerous studies and regulatory agencies affirming its non-toxic and non-irritating properties. Unlike many chemical disinfectants, hypochlorous acid does not produce harmful fumes or residues, making it safe for use in enclosed spaces and around sensitive populations, including children and pets.
In healthcare settings, hypochlorous acid is used extensively for wound care and skin disinfection, highlighting its safety for direct contact with human tissues. Its gentle nature allows for frequent application without causing irritation or adverse reactions, making it suitable for individuals with sensitive skin or allergies.
Despite its safety, it is important to use hypochlorous acid as directed and in appropriate concentrations to ensure its effectiveness and prevent potential degradation. When used correctly, hypochlorous acid is a safe and reliable option for achieving high levels of sanitation and hygiene across various applications.
Production Methods of Hypochlorous Acid
Hypochlorous acid can be produced through several methods, with the most common being electrolysis of saltwater. This process involves passing an electric current through a solution of sodium chloride (common salt) and water, resulting in the formation of hypochlorous acid and sodium hydroxide. This method is widely used due to its simplicity, efficiency, and cost-effectiveness.
Another method of producing hypochlorous acid involves the reaction of chlorine gas with water. This approach is typically used in industrial settings where large quantities of hypochlorous acid are required. The chemical reaction between chlorine and water yields hypochlorous acid and hydrochloric acid, with the former being the desired product for disinfection purposes.
Regardless of the production method, ensuring the purity and stability of hypochlorous acid is crucial for its effectiveness. Proper storage and handling are necessary to maintain its potency and prevent degradation, ensuring reliable performance in sanitation applications.
Comparison with Other Disinfectants
When compared to other disinfectants, hypochlorous acid offers several distinct advantages. One of the most notable is its safety profile, as it is non-toxic and non-irritating, unlike many chemical disinfectants that can cause harm to humans and the environment. This safety makes hypochlorous acid suitable for a wide range of applications, including those involving direct contact with skin and surfaces.
In terms of efficacy, hypochlorous acid is comparable to or even superior to many traditional disinfectants. Its rapid action and broad-spectrum antimicrobial properties allow it to effectively kill bacteria, viruses, and fungi, providing reliable sanitation in various settings. Additionally, hypochlorous acid does not contribute to antibiotic resistance, a growing concern with the use of certain chemical disinfectants.
Despite its advantages, hypochlorous acid may have limitations in terms of stability and shelf life, requiring proper storage and handling to maintain its effectiveness. However, its benefits in terms of safety, efficacy, and environmental impact make it a compelling choice for many sanitation and disinfection needs.
Storage and Stability Considerations
The stability of hypochlorous acid is a key consideration for its effective use. As a reactive compound, hypochlorous acid can degrade over time, particularly when exposed to light, heat, and air. To maintain its potency, it is important to store hypochlorous acid in a cool, dark, and airtight container, away from direct sunlight and sources of heat.
Proper storage conditions can significantly extend the shelf life of hypochlorous acid, ensuring that it remains effective for its intended applications. Additionally, using stabilized forms of hypochlorous acid, which contain additives to enhance stability, can further improve its longevity and performance.
Understanding the factors that affect the stability of hypochlorous acid is crucial for maximizing its effectiveness and ensuring reliable sanitation results. By adhering to recommended storage practices, users can ensure that hypochlorous acid retains its antimicrobial properties and delivers the desired level of hygiene and safety.
Chemical Reactions Involving Hypochlorous Acid
Hypochlorous acid is involved in a variety of chemical reactions, primarily due to its role as an oxidizing agent. One of its most significant reactions is its ability to oxidize and kill microbial cells, a process that involves the denaturation of proteins and the destruction of cellular components.
In addition to its antimicrobial actions, hypochlorous acid participates in chemical reactions with organic and inorganic compounds. For instance, it can react with ammonia and amines to form chloramines, a class of compounds with disinfectant properties. This reaction is commonly utilized in water treatment processes to maintain water quality and safety.
Hypochlorous acid can also undergo disproportionation reactions, where it decomposes into chloride and chlorate ions. These reactions can occur under certain conditions, such as high temperatures or extreme pH levels, and can affect the stability and effectiveness of hypochlorous acid in disinfection applications.
Future Prospects of Hypochlorous Acid
The future prospects of hypochlorous acid are promising, as its applications continue to expand across various industries. With increasing awareness of the importance of hygiene and sanitation, hypochlorous acid is poised to play a crucial role in ensuring public health and safety.
Advancements in technology and production methods are expected to enhance the availability and affordability of hypochlorous acid, making it accessible to a wider range of users. Additionally, ongoing research into its antimicrobial properties and potential applications may uncover new uses for hypochlorous acid, further solidifying its position as a versatile and effective disinfectant.
As environmental concerns and sustainability become more prominent, hypochlorous acid's eco-friendly nature will likely drive its adoption in industries seeking to implement environmentally responsible practices. Its combination of safety, efficacy, and environmental impact positions hypochlorous acid as a valuable tool for addressing sanitation challenges in the future.
Frequently Asked Questions
1. What is the chemical formula for hypochlorous acid?
The chemical formula for hypochlorous acid is HOCl, representing the combination of hydrogen, oxygen, and chlorine.
2. How does hypochlorous acid kill bacteria and viruses?
Hypochlorous acid kills bacteria and viruses by penetrating their cell walls and causing oxidative damage to essential cellular components, leading to cell death.
3. Is hypochlorous acid safe for use on skin?
Yes, hypochlorous acid is safe for use on skin, as it is non-toxic and non-irritating. It is commonly used in wound care and skin disinfection.
4. How is hypochlorous acid produced?
Hypochlorous acid can be produced through electrolysis of saltwater or by reacting chlorine gas with water.
5. What are the environmental benefits of using hypochlorous acid?
Hypochlorous acid is environmentally friendly, breaking down into harmless byproducts and aligning with sustainable practices.
6. Can hypochlorous acid be used in food processing?
Yes, hypochlorous acid is used in food processing to disinfect surfaces, equipment, and food products, ensuring food safety and quality.
Conclusion
Hypochlorous acid, with its chemical formula HOCl, stands as a vital component in the realm of disinfection and sanitation. Its unique properties, derived from its simple yet effective composition, make it an invaluable tool across various industries. From healthcare to food processing, hypochlorous acid offers a safe, effective, and environmentally friendly solution for maintaining hygiene and safety.
As we continue to prioritize health and sustainability, the role of hypochlorous acid is expected to grow, driven by advancements in production methods and an increasing emphasis on eco-friendly practices. Understanding the chemical formula for hypochlorous acid and its implications allows us to appreciate its significance and potential in addressing the sanitation challenges of today and the future.
For more detailed information and further reading on hypochlorous acid, consider visiting reputable chemistry and health resources, such as those provided by the Centers for Disease Control and Prevention (CDC) or the World Health Organization (WHO).