The world of chemistry is filled with fascinating shapes and configurations that govern the behavior and properties of molecules. Among these, the trigonal pyramidal geometry stands out for its unique structure and significant role in determining molecular characteristics. This geometric form is not just a simple arrangement of atoms; it is a fundamental aspect that influences how molecules interact, react, and exist in various states of matter. Understanding trigonal pyramidal geometry can offer insights into the nature of compounds and their applications across different scientific fields.
Trigonal pyramidal geometry is commonly found in molecules where a central atom is bonded to three other atoms, with one lone pair of electrons. This arrangement results in a three-dimensional shape resembling a pyramid with a triangular base. The presence of the lone pair on the central atom is crucial, as it creates an asymmetry that affects the molecule’s polarity and reactivity. This geometry is often observed in compounds containing nitrogen, phosphorus, and sulfur, where the lone pair plays a pivotal role in chemical reactions and bonding characteristics.
Delving deeper into the world of trigonal pyramidal structures reveals its applications and importance in various scientific domains. From understanding the behavior of ammonia (NH3) to exploring the intricacies of phosphine (PH3), the trigonal pyramidal geometry provides a foundation for studying molecular interactions and properties. This article aims to explore the nuances of trigonal pyramidal geometry, its significance in chemistry, and the scientific principles that underpin its formation and effects.
Read also:Is It Safe To Eat Unripe Avocado A Guide To Understanding Avocado Ripeness
Table of Contents
- What is Trigonal Pyramidal Geometry?
- Formation and Characteristics
- Molecular Examples of Trigonal Pyramidal Geometry
- The Role of Lone Pairs in Trigonal Pyramidal Geometry
- How Does It Affect Molecular Properties?
- Trigonal Pyramidal vs Trigonal Planar: What's the Difference?
- Uses in Real-World Applications
- Significance in Organic Chemistry
- Implications in Industrial Processes
- Educational Importance of Understanding Trigonal Pyramidal Geometry
- Frequently Asked Questions
- Conclusion
What is Trigonal Pyramidal Geometry?
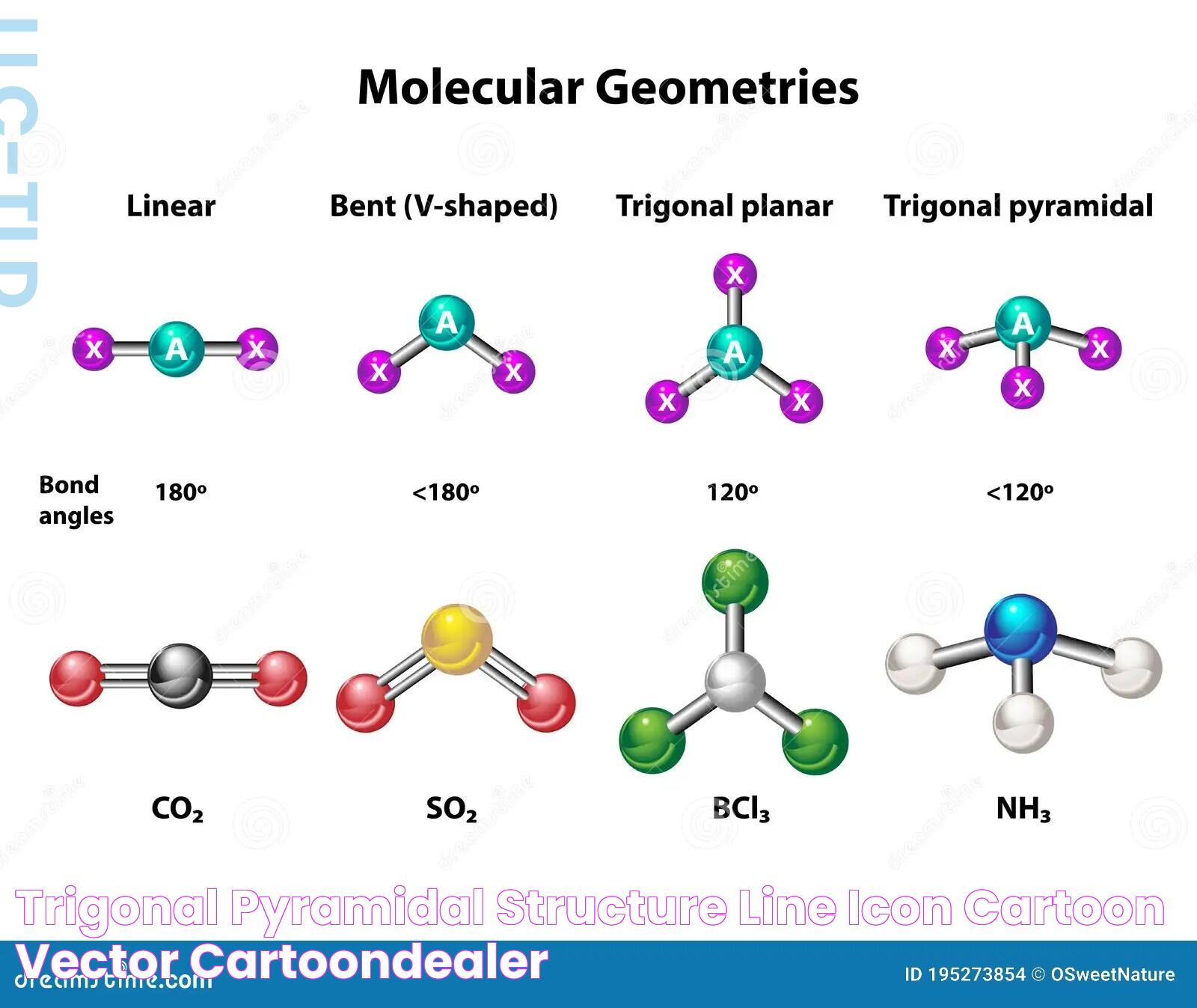
Trigonal pyramidal geometry is a molecular shape that arises when a central atom is bonded to three other atoms and possesses a lone pair of electrons. This geometry is distinct from the more symmetrical trigonal planar shape, as the lone pair exerts a repulsive force, pushing the bonded atoms away and creating a three-dimensional pyramidal structure. The angles between the bonds in a trigonal pyramidal molecule are typically less than the 120 degrees found in a trigonal planar molecule due to this repulsion.
The concept of trigonal pyramidal geometry is rooted in valence shell electron pair repulsion (VSEPR) theory, which seeks to explain the spatial arrangement of atoms in a molecule based on electron pair interactions. According to VSEPR theory, electron pairs, whether in bonds or as lone pairs, will arrange themselves as far apart as possible to minimize repulsion. In a trigonal pyramidal molecule, this results in a shape where the lone pair occupies more space than a bonded pair, leading to a pyramid-like configuration.
Key Characteristics
- Presence of a central atom bonded to three other atoms
- One lone pair of electrons influencing molecular shape
- Bond angles typically less than 109.5 degrees due to lone pair repulsion
- Asymmetrical shape leading to potential polarity
Formation and Characteristics
The formation of a trigonal pyramidal molecule is heavily influenced by the electronic configuration of the central atom. Atoms such as nitrogen, phosphorus, and sulfur are common central atoms in trigonal pyramidal structures due to their ability to accommodate a lone pair of electrons while forming three covalent bonds. The hybridization of the central atom also plays a crucial role, with sp3 hybridization being a typical scenario for trigonal pyramidal geometry.
Hybridization involves the mixing of atomic orbitals to form new hybrid orbitals that can form sigma bonds with other atoms. In the case of a trigonal pyramidal molecule, the central atom undergoes sp3 hybridization, resulting in four hybrid orbitals—three forming sigma bonds with adjacent atoms and one accommodating the lone pair. This arrangement ensures maximum separation between the electron pairs, minimizing repulsive forces.
Lone Pair Effect
- Lone pairs occupy more space than bonded pairs
- Greater repulsion from lone pairs alters bond angles
- Makes the molecule polar due to asymmetrical shape
Molecular Examples of Trigonal Pyramidal Geometry
Trigonal pyramidal geometry is observed in several well-known molecules, each exhibiting distinct characteristics due to the lone pair-induced asymmetry. Ammonia (NH3) is perhaps the most widely recognized example of a trigonal pyramidal molecule. In NH3, the nitrogen atom is the central atom with three hydrogen atoms bonded to it and a lone pair of electrons. The lone pair's repulsion pushes the hydrogen atoms closer together, resulting in a bond angle of about 107 degrees, slightly less than the tetrahedral angle of 109.5 degrees.
Another example is phosphine (PH3), where the phosphorus atom is surrounded by three hydrogen atoms and a lone pair. Similar to ammonia, the lone pair in phosphine influences the molecular shape, leading to a trigonal pyramidal configuration. Despite the similar geometry, PH3 is less polar than NH3 due to the lower electronegativity difference between phosphorus and hydrogen compared to nitrogen and hydrogen.
Read also:Osrs Wyrm A Deep Dive Into The Mystical Creatures Of Old School Runescape
Other Examples
- Arsenic trihydride (AsH3)
- Sulfur trioxide anion (SO32-)
- Nitrogen trihalides (NF3, NCl3, etc.)
The Role of Lone Pairs in Trigonal Pyramidal Geometry
Lone pairs of electrons are a defining feature of trigonal pyramidal geometry, significantly impacting the shape and properties of the molecule. Unlike bonded pairs, lone pairs are not shared between atoms and occupy more space around the central atom. This increased spatial requirement leads to greater electron pair repulsion, which in turn affects the bond angles and overall geometry of the molecule.
The presence of a lone pair in a trigonal pyramidal molecule also contributes to its polarity. Since the lone pair is not evenly distributed around the central atom, it creates a dipole moment, making the molecule polar. This polarity has important implications for the molecule's interactions with other compounds, solubility in different solvents, and participation in chemical reactions.
Influence on Reactivity
- Polar molecules can engage in dipole-dipole interactions
- Increased reactivity due to lone pair availability
- Polar molecules have specific solubility characteristics
How Does It Affect Molecular Properties?
The trigonal pyramidal geometry significantly influences the physical and chemical properties of a molecule. One of the most noticeable effects is on the molecule's polarity, which arises due to the asymmetrical distribution of electron density caused by the lone pair. Polarity affects how a molecule interacts with other substances, determining its solubility, boiling and melting points, and ability to form hydrogen bonds.
For instance, the polar nature of ammonia allows it to dissolve readily in water and form strong hydrogen bonds, contributing to its relatively high boiling point compared to nonpolar molecules of similar size. In contrast, less polar molecules like phosphine exhibit lower boiling points and different solubility characteristics due to weaker intermolecular forces.
Impact on Chemical Reactions
- Polar molecules can act as nucleophiles in reactions
- Lone pairs offer sites for coordination with metal ions
- Polarity influences reaction mechanisms and pathways
Trigonal Pyramidal vs Trigonal Planar: What's the Difference?
While trigonal pyramidal and trigonal planar geometries might sound similar, they are distinct in terms of structure and properties. A trigonal planar molecule consists of a central atom bonded to three other atoms with no lone pairs, resulting in a flat, symmetrical arrangement with 120-degree bond angles. In contrast, a trigonal pyramidal molecule has a lone pair, leading to a three-dimensional, asymmetrical shape with bond angles less than 109.5 degrees.
The lack of a lone pair in trigonal planar molecules results in nonpolar characteristics, assuming the bonded atoms are identical. This symmetry affects how these molecules interact with other substances, often leading to different solubility and reactivity patterns compared to their trigonal pyramidal counterparts. Understanding these differences is crucial for predicting the behavior of molecules in various chemical contexts.
Comparative Analysis
- Trigonal planar: 120-degree bond angles, symmetrical, nonpolar
- Trigonal pyramidal:
- Different reactivity and interaction characteristics
Uses in Real-World Applications
The trigonal pyramidal geometry is more than a theoretical concept; it has practical implications in a variety of real-world applications. For instance, ammonia, a classic example of a trigonal pyramidal molecule, is widely used as a fertilizer, refrigerant, and cleaning agent due to its polar nature and ability to form hydrogen bonds. Its geometry plays a critical role in these applications, influencing its solubility, reactivity, and interaction with other substances.
In the pharmaceutical industry, understanding the geometry of molecules such as drugs is essential for predicting their interactions with biological targets. The trigonal pyramidal shape can affect how a drug molecule binds to receptors, impacting its efficacy and safety profile. Similarly, in materials science, the geometry of molecules influences the properties of polymers and other synthetic materials, guiding their design and application.
Industrial Relevance
- Ammonia in fertilizers and industrial cleaning
- Drug design and interaction predictions
- Material properties and polymer design
Significance in Organic Chemistry
In organic chemistry, trigonal pyramidal geometry is a key concept that influences the behavior and reactivity of various compounds. Many organic molecules contain nitrogen or phosphorus atoms that adopt this geometry, affecting their chemical properties and interactions. For example, amines, which are organic derivatives of ammonia, exhibit trigonal pyramidal geometry and are involved in numerous chemical reactions and processes.
The lone pair on the nitrogen atom in amines makes them nucleophilic, allowing them to participate in nucleophilic substitution reactions. This property is leveraged in organic synthesis to form new carbon-nitrogen bonds, crucial for building complex organic molecules. Additionally, the geometry of these compounds affects their acidity and basicity, influencing their role in acid-base reactions.
Functional Group Chemistry
- Amines as nucleophiles in organic synthesis
- Influence on acidity and basicity of compounds
- Role in forming carbon-nitrogen bonds
Implications in Industrial Processes
The trigonal pyramidal geometry is not only important in academic settings but also has significant implications in industrial processes. The reactivity and polarity of trigonal pyramidal molecules make them valuable in various chemical industries. For example, ammonia is a critical component in the production of fertilizers through the Haber-Bosch process, which relies on the unique properties of the molecule to synthesize ammonia from nitrogen and hydrogen gases.
In addition to fertilizers, ammonia is used in the production of explosives, textiles, and plastics, highlighting the diverse applications of trigonal pyramidal molecules. Understanding the geometry of these compounds is essential for optimizing industrial processes and improving the efficiency and sustainability of chemical production.
Industrial Applications
- Fertilizer production via the Haber-Bosch process
- Explosives and chemical manufacturing
- Textile and plastic production
Educational Importance of Understanding Trigonal Pyramidal Geometry
Teaching and understanding trigonal pyramidal geometry is vital in education, as it lays the groundwork for comprehending more complex chemical concepts. This geometry is a fundamental part of molecular structure education, providing students with insights into molecular shapes, bond angles, and electron pair interactions. Mastering these concepts is crucial for students aspiring to pursue careers in chemistry, biology, and related fields.
Educational curricula often include laboratory experiments and molecular modeling exercises to help students visualize and understand trigonal pyramidal geometry. These practical experiences reinforce theoretical knowledge and enhance students' ability to predict and explain molecular behavior, fostering a deeper appreciation for the intricacies of chemical structures.
Pedagogical Strategies
- Use of molecular models and simulations
- Hands-on laboratory experiments
- Integration of geometry in chemical education
Frequently Asked Questions
1. What molecules exhibit trigonal pyramidal geometry?
Molecules such as ammonia (NH3), phosphine (PH3), and nitrogen trihalides (e.g., NF3) exhibit trigonal pyramidal geometry due to the presence of a lone pair on the central atom.
2. How does trigonal pyramidal geometry affect molecular polarity?
The asymmetrical arrangement of atoms and lone pairs in a trigonal pyramidal molecule leads to an uneven distribution of electron density, resulting in molecular polarity.
3. What is the difference between trigonal pyramidal and trigonal planar geometries?
Trigonal pyramidal geometry has a lone pair and a three-dimensional shape, while trigonal planar geometry is flat and symmetrical with no lone pairs.
4. Why is ammonia considered a trigonal pyramidal molecule?
Ammonia has a nitrogen atom bonded to three hydrogen atoms with a lone pair, creating a trigonal pyramidal shape due to lone pair repulsion.
5. How does lone pair repulsion influence bond angles in trigonal pyramidal molecules?
Lone pair repulsion reduces bond angles in trigonal pyramidal molecules to less than the tetrahedral angle of 109.5 degrees, typically around 107 degrees.
6. Can trigonal pyramidal geometry be found in organic molecules?
Yes, many organic molecules such as amines exhibit trigonal pyramidal geometry due to the presence of a nitrogen or phosphorus atom with a lone pair.
Conclusion
Trigonal pyramidal geometry is a fundamental concept in chemistry that influences the structure, properties, and behavior of molecules. Its unique shape, caused by the presence of a lone pair, leads to asymmetrical electron distribution, affecting molecular polarity, reactivity, and interactions. Understanding trigonal pyramidal geometry provides valuable insights into the nature of compounds and their applications across various scientific and industrial fields. From ammonia's role in fertilizers to the significance of amines in organic synthesis, the implications of this geometry are vast and impactful, underscoring its importance in both academic and practical contexts.