In the fascinating world of chemistry, the arrangement of atoms in a molecule can determine its properties, reactivity, and functionality. One such arrangement that captures the curiosity of many is the trigonal pyramidal bond angle. This specific geometric configuration plays a crucial role in determining the molecular shape and its corresponding behavior in various chemical reactions. By understanding the intricacies of the trigonal pyramidal bond angle, one can gain deeper insights into the fundamental principles of molecular geometry.
The trigonal pyramidal bond angle is not just a theoretical concept; it has practical implications in everyday life, from the behavior of water molecules to the structure of complex biomolecules. The unique three-sided pyramid shape, with a central atom bonded to three other atoms and a lone pair of electrons, creates a distinct angle that influences the molecule's overall shape. This angle is critical in determining how molecules interact with each other, affecting everything from the flavor of food to the efficacy of pharmaceuticals.
Delving into the world of trigonal pyramidal bond angles offers a window into the microscopic interactions that define the macroscopic world. Understanding these angles requires a grasp of concepts such as electron pair repulsion, hybridization, and molecular orbital theory. With this knowledge, one can appreciate the elegance and complexity of molecular structures and their role in the world around us. This article aims to provide a comprehensive overview of the trigonal pyramidal bond angle, its significance, and its applications across various fields of study.
Read also:Fortis Fortuna Adiuvat Discover Its Timeless Significance
Table of Contents
- What is Trigonal Pyramidal Bond Angle?
- Molecular Geometry and Bond Angles
- Importance of Trigonal Pyramidal Structures
- How Does Trigonal Pyramidal Angle Affect Chemical Properties?
- Examples of Molecules with Trigonal Pyramidal Shape
- Understanding Electron Pair Repulsion
- Role of Hybridization in Trigonal Pyramidal Geometry
- Comparison with Other Molecular Shapes
- Applications in Chemistry and Beyond
- Impact on Biological Molecules
- How Does Temperature Affect Trigonal Pyramidal Bond Angles?
- Trigonal Pyramidal Bond Angle in Industry
- Common Misconceptions About Trigonal Pyramidal Angles
- Frequently Asked Questions
- Conclusion
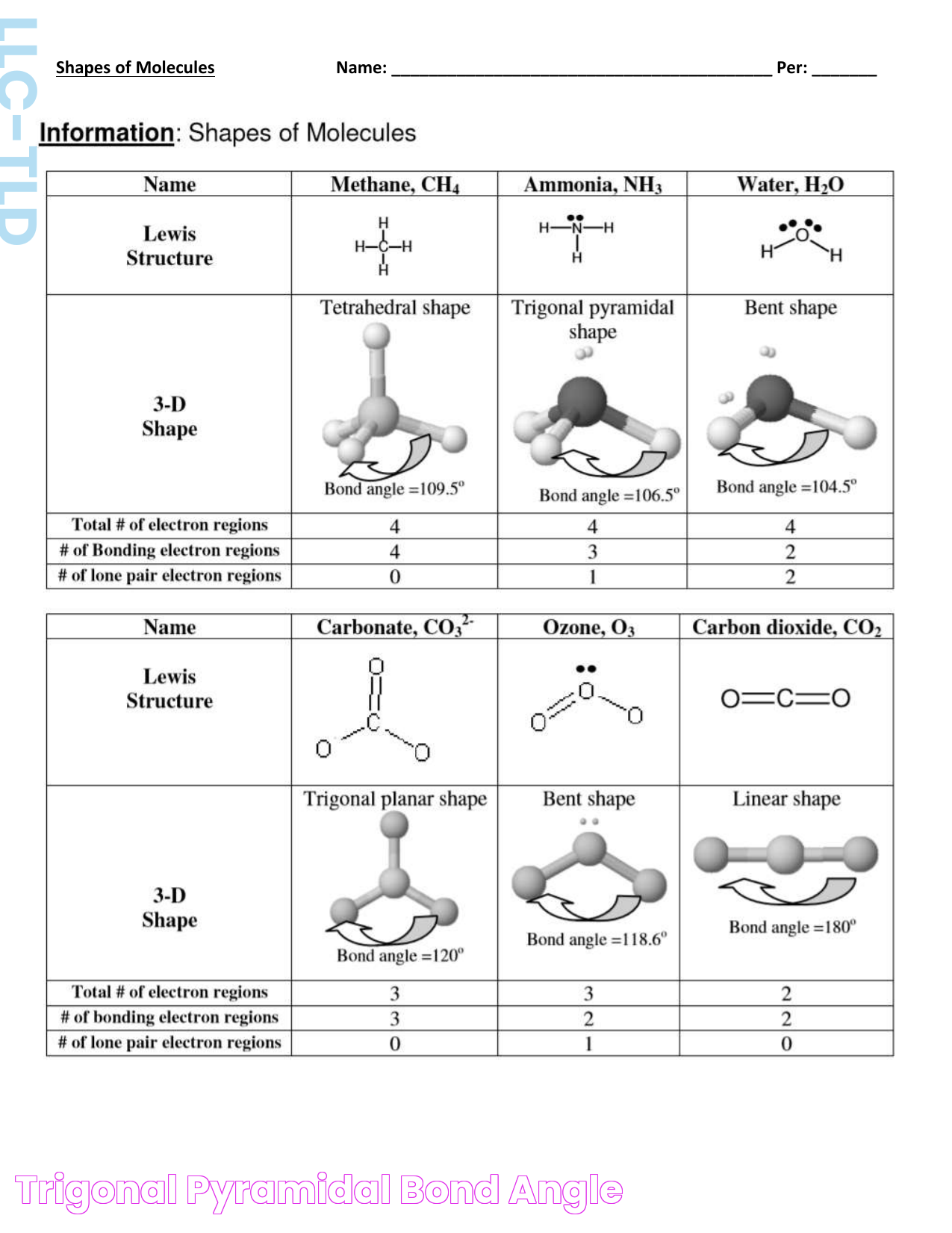
What is Trigonal Pyramidal Bond Angle?
The trigonal pyramidal bond angle is a specific angle formed between the bonds in a trigonal pyramidal molecule. This molecular shape is characterized by a central atom bonded to three other atoms and possessing a lone pair of electrons. The presence of the lone pair leads to a distortion from the ideal tetrahedral angle, resulting in a typically smaller bond angle of around 107 degrees.
Molecular Geometry and Bond Angles
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. Bond angles are crucial in determining a molecule's shape, influencing its chemical and physical properties. The VSEPR (Valence Shell Electron Pair Repulsion) theory helps explain these arrangements, including the formation of the trigonal pyramidal bond angle.
Importance of Trigonal Pyramidal Structures
Trigonal pyramidal structures are significant because they influence how molecules interact with each other. These structures are found in many important molecules, such as ammonia (NH3), where the lone pair of electrons on the nitrogen atom affects the molecule's polarity and reactivity.
How Does Trigonal Pyramidal Angle Affect Chemical Properties?
The angle in trigonal pyramidal molecules impacts their dipole moments, solubility, and reactivity. Molecules with this configuration often have unique properties that make them suitable for specific chemical reactions and applications.
Examples of Molecules with Trigonal Pyramidal Shape
Several molecules exhibit a trigonal pyramidal shape, such as ammonia (NH3), phosphorus trichloride (PCl3), and sulfur trioxide (SO3). These molecules have significant roles in various chemical processes and industries.
Understanding Electron Pair Repulsion
Electron pair repulsion is a concept that helps explain the shape of molecules, including the trigonal pyramidal bond angle. The lone pair of electrons exerts repulsion on the bonded pairs, causing the bond angles to adjust accordingly.
Read also:Height Trends Average Height Of Women In The Philippines
Role of Hybridization in Trigonal Pyramidal Geometry
Hybridization involves the mixing of atomic orbitals to form new hybrid orbitals. In trigonal pyramidal geometry, sp3 hybridization occurs, where one s orbital mixes with three p orbitals, influencing the bond angle and molecular shape.
Comparison with Other Molecular Shapes
Trigonal pyramidal structures can be compared to other molecular shapes, such as tetrahedral and bent shapes. These comparisons help in understanding the diversity of molecular geometries and their implications in chemistry.
Applications in Chemistry and Beyond
Molecules with trigonal pyramidal shapes are used in various applications, from industrial processes to pharmaceuticals. Understanding their properties helps in designing more effective chemical products and solutions.
Impact on Biological Molecules
Trigonal pyramidal bond angles play a role in the structure and function of biological molecules, such as proteins and enzymes. These angles influence how biomolecules interact with each other and with other substances.
How Does Temperature Affect Trigonal Pyramidal Bond Angles?
Temperature can affect the bond angles in trigonal pyramidal molecules by influencing the kinetic energy of atoms and the strength of intermolecular forces. This, in turn, can alter the molecule's properties and behavior.
Trigonal Pyramidal Bond Angle in Industry
In the industrial sector, understanding the trigonal pyramidal bond angle is essential for the development of chemicals and materials with specific properties. This knowledge aids in optimizing production processes and enhancing product performance.
Common Misconceptions About Trigonal Pyramidal Angles
There are several misconceptions about trigonal pyramidal angles, such as confusing them with other molecular geometries or misunderstanding their effects on molecular properties. Clarifying these misconceptions helps in accurately interpreting chemical concepts.
Frequently Asked Questions
- What causes a trigonal pyramidal shape in molecules?
- How is the trigonal pyramidal bond angle different from the tetrahedral angle?
- Can the trigonal pyramidal bond angle change?
- What are some examples of trigonal pyramidal molecules in nature?
- Why is understanding trigonal pyramidal bond angle important?
- How do lone pairs affect the trigonal pyramidal bond angle?
Trigonal pyramidal shape arises due to the presence of a lone pair of electrons on the central atom, which repels the bonded atoms, causing a distortion from the tetrahedral structure.
The trigonal pyramidal bond angle is typically around 107 degrees, which is less than the ideal tetrahedral angle of 109.5 degrees, due to the repulsion of the lone pair of electrons.
Yes, the bond angle can change depending on factors such as temperature, pressure, and the presence of other atoms or molecules that influence electron pair repulsion.
Examples include ammonia (NH3), phosphorus trichloride (PCl3), and sulfur trioxide (SO3), which are found in various natural and industrial processes.
Understanding this bond angle is crucial for predicting molecular behavior, designing chemical reactions, and developing materials with specific properties.
Lone pairs exert greater repulsion compared to bonded pairs, causing the bond angle to decrease and the molecular shape to become trigonal pyramidal.
Conclusion
The trigonal pyramidal bond angle is a fundamental concept in chemistry, influencing molecular geometry and properties. By understanding this angle, one can gain insights into the behavior of molecules in various chemical reactions and applications. This knowledge is essential for advancing our understanding of molecular interactions and designing innovative solutions in science and industry.